Infrared Spectra. Free Download of FTIR Library
Download free infrared spectra library – FTIR Library (go
to page bottom).

![]()
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
Following text is a basic explanation for beginners in
infrared spectroscopy.
|
Infrared spectra are something like „fingerprints“ of
molecules. The same molecules have the same infrared spectra, different molecules
have different infrared spectra. You put a sample into an instrument called infrared
spectrometer (IR, FTIR spectrometer) and in a few seconds you get a spectrum. In the instrument, the sample is irradiated by infrared
radiation (heat, small range of electromagnetic spectrum). The sample absorbs
some typical wavelengths and the detector detects the wavelength which were
not absorbed by the sample. The result is called infrared (IR or FTIR) spectrum. Infrared spectra are used in chemistry, mostly in
analytical chemistry. Typical infrared spectrum looks like this (Picture 1): |
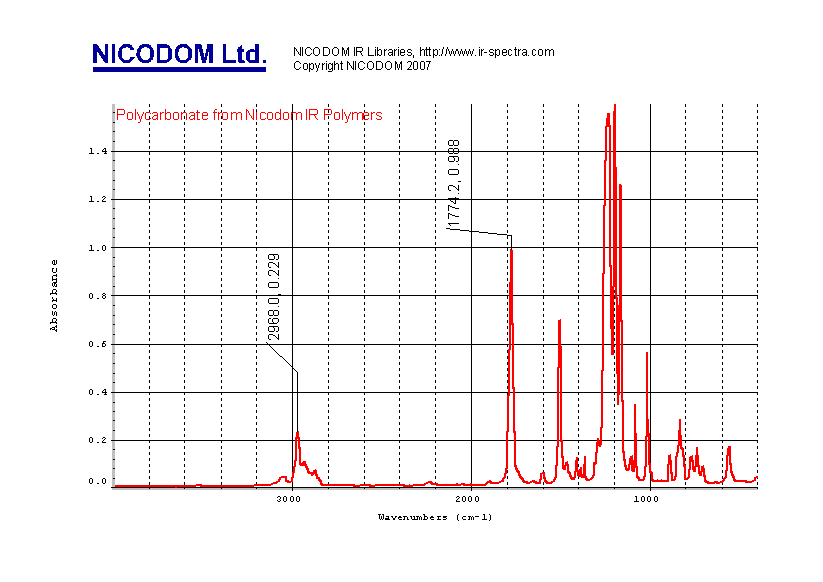
|
Infrared spectra are curves. The final infrared spectrum
is a ratio of sample spectrum and air spectrum (background). The X-axis (peak position) gives you information about the wavelength (usually wavenumber)
and is usually presented in units called „cm-1“ = wavenumbers (number of
waves in 1 centimeter). Typical wavenumber range by infrared spectrum is
4000-400 cm-1 (wavelength in micrometers would be 2.5-25 micrometers) The Y-axis (peak intensity) gives you information about
how much the sample absorbs the energy. The typical Y units are Transmittance (%T - …peaks go
down) or Absorbance (logarithmic scale, peaks go up) units. Transmittance ranges from 0-100%. Transmitance 100% - sample does not absorb any energy of
this wavelength, all energy (100%) falls on detector Transmitance 100% - sample absorbs 50% energy of this
wavelength, 50% of energy falls on detector Transmitance
0% - sample absorbs complete energy of this wavelength, no (0%) energy
falls on detector Absorbance ranges from 0 to infinity. If Absorbance A=0,
sample does not absorb any energy of this wavelength, all energy falls on
detector. If you are not familiar with logarithmic scale, than
note that: A=0 is the same as %T=100% A=1 is the same as %T=10%. A=2 is the same as %T=1%. A=3 is the same as %T=0.1% etc. Absorbance is widely used because the Y- scale is
proportional to component concentration. By your spectrum we recommend to
take in account absorbance values 0-1 or maximally 0-2. Although your
software will show higher absorbance values, you should not trust to values
higher than 2 absorbance units. This is because the detector sensitivity goes
rapidly down if low energy falls on the detector Other Y units can be reflectance etc. About the spectrum on picture 1 you can tell following:
etc. |
The same spectrum in transmittance units looks like this
(Picture 2):
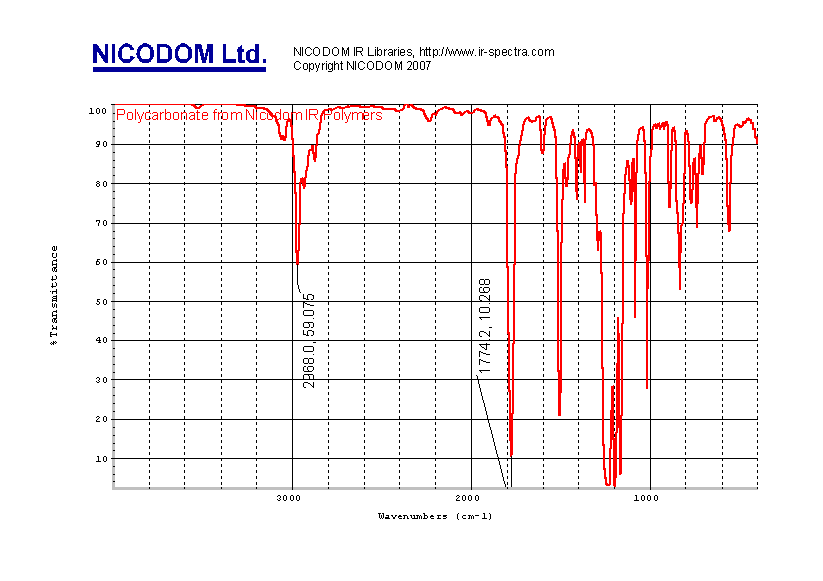
Valuable
FTIR spectroscopy links and videos:
http://en.wikipedia.org/wiki/Infrared_spectroscopy
https://en.wikipedia.org/wiki/Fourier-transform_infrared_spectroscopy
http://www.cem.msu.edu/~reusch/VirtualText/Spectrpy/InfraRed/infrared.htm
http://wwwchem.uwimona.edu.jm/spectra/IRIndex.html
https://jascoinc.com/learning-center/theory/spectroscopy/fundamentals-ftir-spectroscopy/
https://scienceinfo.com/ftir-principle-instrumentation-applications/
https://www.innovatechlabs.com/newsroom/672/stuff-works-ftir-analysis/
https://www.youtube.com/watch?v=xgz8FvgCE-U
https://www.youtube.com/watch?v=KRoWMB3AR3s
https://www.youtube.com/watch?v=9NCaMHlKzOU
….and many other
Measurement
Techniques
You can collect your spectra by transmission techniques
(KBr pellets, Nujol mulls, disposable cards, liquid cells, gas cells…) or
reflection techniques (ATR, diffuse reflectance, specular reflectance…). Transmission techniques (infrared beam goes thru sample)
are classical and will deliver the best quality spectrum, but they are time
consuming and have other problems (sample must be very thin, it is difficult
to use and clean the material which holds the sample - typically KBr etc.) Today 70-90% of new instruments are delivered with an
ATR (= attenuated total reflection) sampling unit. In case of ATR the
infrared beam enters the sample surface and than it reflects back. Collection
by ATR technique does not require any sample preparation. Typical time of the
spectrum collection by ATR together with sample adjustment and crystal
cleaning is 2-5 minutes. Instrument time can be a few seconds only. The
quality of the ATR spectrum is fully sufficient for sample identification (if
it is correctly collected...). If you want to compare your spectrum of your unknown
sample with spectral libraries, than the libraries should be collected by the
same technique as your sample. If you collect by ATR, just use ATR spectral
libraries. If you still collect in transmission (oldfashioned technique),
than old transmission libraries give you better match. The ATR spectrum may
have limitations if you look for trace impurities in your sample and in a few
other very special cases. But the advantages of ATR dominate. Sampling Materials in IR spectroscopy
All chemical compounds absorb in the infrared region.
This means all sampling materials have their own infrared spectrum. In most
cases you need some sampling material, because you cannot put the solid,
liquid or gas sample to the spectrometer without sampling material (polymer
foils are an exception). Sampling material peaks in your spectrum are
unwanted and would cause difficulties by the interpretation. To avoid/remove the unwanted peaks in your spectrum you
have following possibilities:
Typical transmission materials: KBr, NaCl, BaF2,
ZnSe…
Glass or quartz cannot be used – their own absorption is
too strong in IR region. Typical ATR materials:
ZnSe, Si, Diamond, Ge… Peaks in infrared spectra
The reason why you collect infrared spectra is usually
unknown compound identification, identification/quantification of components
in mixture (impurities, additives…), verification of sample identity and
purity etc. An expert with help of supporting computer programs evaluates
peak positions and/or their intensities. The peaks in infrared spectra are caused by absorption
of characteristic frequencies by molecules. Those absorptions are mostly
caused by different vibration/rotation modes of a molecule. Different functional groups in a molecule (like –CH3,
COOH, NH2) exhibit peaks at different
ranges of wavenumbers. The peak position gives you information about
sample quality (organic x inorganic, alifatic x aromatic, which functional
groups are present…) and depends on the symetry of the molecule, atom weight
and bond stenght. Peak intensity
gives you quantitative information and depends on concentration. The qualitative evaluation of infrared spectra can be
done by following two methods. Spectral Interpretation
If an expert looks at the infrared spectrum, he is able
to interpret it. This means, he can estimate what functional groups are
present and in simple cases even establish the chemical composition of the
compound. In the above example, it is well known that the peak at
2968 cm-1 is the -CH3 peak and peak at 1774 cm-1 is the C=O peak. Further we
can see peaks of aromates etc. If you
show this spectrum to an expert on the field of infrared spectra of polymers
who works with polycarbonates, he can
identify a polycarbonate on the first look, because he remembers the spectrum
shape. But he cannot easily identify spectra which he has never seen before. Generally spectral interpretation is easily possible for
simple compounds. But it is time
consuming, expensive, it requires deep knowledge of an expert and is generaly
limited by more complicated compounds. Although computer programs have been
developed for infrared spectra interpretation, this method is used relatively
rarely. Without consultation with expert computerized interpretation
frequently does not bring satisfying results. SEARCH Spectra – Compare with Libraries
Another widely used approach is spectral search –
comparison of unknown spectra with many other spectra and calculation of the
best „hit“ = the most similar spectrum. The main advantage is that spectral SEARCH is fast and
can be used by less qualified personal. To provide spectral search, you need a special software
(„Search Software“) which is usually a part of your instrument. The search
software has a capability of comparing thousands of curves within seconds and
suggest the most similar spectrum. The principle is similar as finger print
search in forensic science. The SEARCH identification is successfull only if the
same spectrum is part of the library you compare with. The absolute values of the hit quality are not so
important. 100% would mean identical spectrum and 0% totally different
spectrum (some software packages have different scaling of the hit
value). In reality you never reach a
hit with value 100%. Sometimes a hit with quality of 70% or even less can be
correct. You must always do a visual comparison of the unknown spectrum with
the library spectrum. It is you who must decide if the spectrum is identical,
not the software. Further you need collections of infrared spectra –
„Infrared Spectral Library“ or „Infrared Spectral Database“. Some spectroscopists create their own spectral
libraries. This is usefull if you work with limited number of compounds, for
example in QC laboratory of company manufacturing 100 products or if you want
to study new or very unique compounds which are not present in any commercial
library. You can download some free spectral libraries but this
is limited (low number of spectra…) If your application is unknown sample identification, if
you study composition of competitive products and if you simply analyze many
different sample kinds, it is usefull to buy a commercial infrared spectra
library. Commercial infrared spectra library is typically a
collection of thousands infrared spectra (curves) in digital format.
Different software and instrument manufacturers use different digital library
formats (you cannot use Bruker library with Thermo software etc.). One from the infrared spectra libraries provider is NICODOM
Ltd. Nicodom offers over 140000
infrared spectra in over 80 libraries in 10 different digital formats. Their webpage gives you detailed description of the
libraries and their prices. You can choose between general big libraries (e.g.
Aldrich – Ichem Library of over 30000 spectra) or smaller special libraries. The choice of suitable spectral library is important and
depends on your application. If your laboratory focuses on one compound type
(e.g. pesticides, dyes, polymers…), you better choose a special library than
the general one. Universities or forensic laboratories usually choose general
libraries. |
Nicodom Ltd. offers a free IR spectra library, go to their
download section:
Download free
infrared spectra library compatible with your format
(10 different formats, information about over 140000 IR
spectra in over 80 databases.)
---------------------------------------------------------------------------------------------
Created by NICODOM Copuright NICODOM 2009-2025